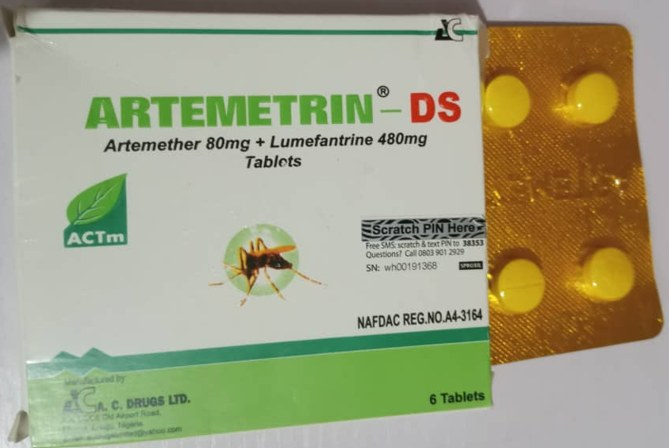
The National Agency for Food and Drug Administration and Control (NAFDAC) has alerted the public about the sale of confirmed substandard and falsified medications, specifically ARTEMETRIN DS and CIPROFIT 500, in Nigeria. The ARTEMETRIN DS (Artemether/Lumefantrine) tablets (80mg/480mg) are labeled as manufactured by A.C. DRUGS Ltd, Emene, Enugu State, while CIPROFIT 500 (Ciprofloxacin Tablet USP 500mg) is labeled as manufactured by Impact Pharmaceutical Ltd, Emene, Enugu State.
Initial Thin-Layer Chromatography (TLC) testing indicated irregularities in both products, prompting further analysis at a WHO-prequalified laboratory. The High-Performance Liquid Chromatography (HPLC) assay revealed alarming discrepancies: ARTEMETRIN DS contained only 59.2% Artemether and 71.2% Lumefantrine, far below the expected 90–110% range, while CIPROFIT 500 contained merely 5.7% of Ciprofloxacin.
NAFDAC emphasized that both products were purchased from “licensed vendors and wholesalers,” yet they do not appear in the agency’s registered products database, and the registration numbers on the packaging are falsified. Consumers are urged to immediately cease the sale or use of these medications and return any stock to the nearest NAFDAC office. Anyone who has ingested the products and experienced adverse reactions is strongly advised to seek prompt medical attention.
Healthcare professionals and members of the public are encouraged to report any suspicion of substandard or falsified medicines to the nearest NAFDAC office, by calling 0800-162-3322, or via email at sf.alert@nafdac.gov.ng.
The alert underscores the critical need for vigilance in pharmaceutical supply chains, highlighting the dangers of counterfeit medicines to public health and the importance of regulatory compliance in safeguarding patients.
Reported by Stone Reporters News 📩 info@stonereportersnews.com | 🌍 stonereportersnews.com | 📘 Facebook: Stone Reporters | 🐦 X (Twitter): @StoneReportNews


Add comment
Comments