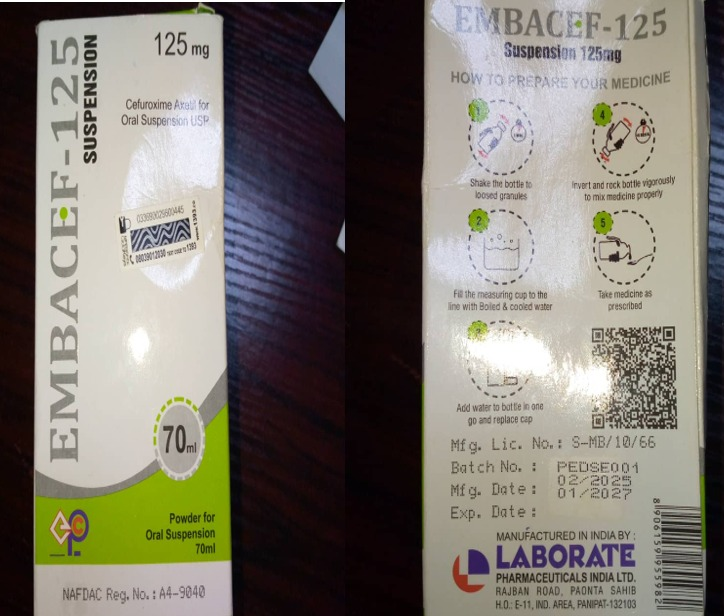
The National Agency for Food and Drug Administration and Control (NAFDAC) has ordered Embassy Pharmaceutical and Chemicals Ltd, Lagos, and its manufacturer, Laborate Pharmaceutical India, to recall Embacef 125 Powder for Oral Suspension after reports of substandard quality.
The directive follows a complaint received in Ekiti State, where two bottles of the reconstituted suspension were reported to have caked after the first day of use. Investigations by NAFDAC confirmed the concerns, prompting the agency to take precautionary measures to protect public health.
Embacef 125, containing the antibiotic Cefuroxime Axetil, is used to treat bacterial infections including bronchitis, skin infections, and urinary tract infections. NAFDAC warned that the use of substandard antibiotics can worsen health conditions and contribute to antibiotic resistance.
Consumers and healthcare professionals are urged to report any suspicion of substandard or falsified medicines to the nearest NAFDAC office, via the hotline 0800-162-3322, or by email at sf.alert@nafdac.gov.ng.
📩 For more updates, visit stonereportersnews.com
🌍 Contact: info@stonereportersnews.com
📘 Facebook: Stone Reporters | 🐦 X (Twitter): @StoneReportNews


Add comment
Comments